Code
# Paths
library(here)
# Multivariate analysis
library(ade4)
library(adegraphics)
# Matrix algebra
library(expm)
# Plots
library(CAnetwork)This is an extension of reciprocal scaling defined for correspondence analysis by Thioulouse and Chessel (1992) to canonical correspondence analysis.
Here, we start from two matrices:
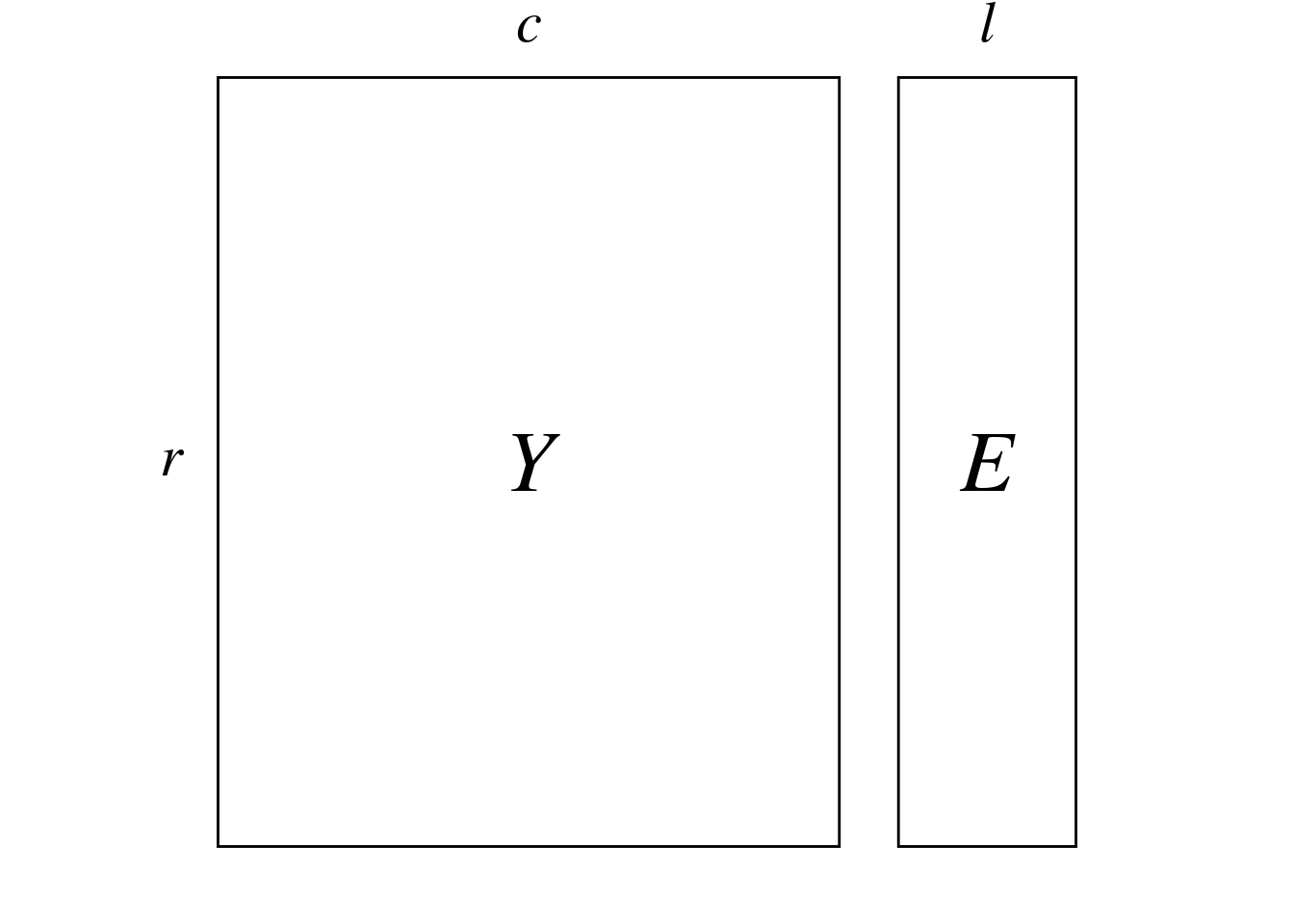
We can compute reciprocal scaling score using CCorA and CCA scores.
From canonical correlation analysis We perform the CCorA of tables \(E_{infl}\) and \(Y_{infl}\). The two sets of scores give the correspondences in sites and species space.
From CCA We can show that\(scoreC = V\) (c1) and \(scoreR = Z2\) (l1).
To perform the canonical correlation analysis, we compute the inflated tables \(R\) (\(n_{\bar{0}} \times l\)) and \(C\) (\(n_{\bar{0}} \times c\)) from \(Y\) (\(r \times c\)) and \(E\) (\(r \times l\)). The difference with CA is that we use \(E\) instead of \(Y\) to compute the inflated table \(R\). \(R\) is equivalent to \(E\) where rows of \(E\) are duplicated as many times as there are correspondences in \(Y\).
# Transform matrix to count table
P <- Y/sum(Y)
Pfreq <- as.data.frame(as.table(P))
colnames(Pfreq) <- c("row", "col", "Freq")
# Remove the cells with no observation
Pfreq0 <- Pfreq[-which(Pfreq$Freq == 0),]
Pfreq0$colind <- match(Pfreq0$col, colnames(Y)) # match index and species names
Pfreq0$col <- factor(Pfreq0$col)
Pfreq0$row <- factor(Pfreq0$row)
pi_ <- rowSums(P)
p_j <- colSums(P)We take the frequency table defined before and use it to compute the inflated tables (with weights):
Below are the first lines of tables \(R\) and \(C\):
| location | elevation | patch_area | perc_forests | perc_grasslands | ShannonLandscapeDiv |
|---|---|---|---|---|---|
| 0 | 10 | 6.28 | 7.7882 | 67.7785 | 0.232 |
| 0 | 30 | 7.92 | 16.4129 | 43.4066 | 0.274 |
| 0 | 430 | 83.24 | 24.4526 | 28.4995 | 0.274 |
| 0 | 420 | 140.83 | 41.9966 | 34.2412 | 0.260 |
| 0 | 400 | 140.83 | 41.9966 | 34.2412 | 0.260 |
| 0 | 500 | 0.50 | 7.5445 | 67.0780 | 0.240 |
| 0 | 470 | 7.84 | 19.3087 | 56.4031 | 0.253 |
| 0 | 110 | 21.15 | 23.4668 | 49.8908 | 0.262 |
| 0 | 460 | 24.82 | 21.3932 | 57.0430 | 0.243 |
| 0 | 450 | 3.11 | 4.8285 | 73.7518 | 0.216 |
| 0 | 40 | 6.66 | 7.4729 | 66.0061 | 0.240 |
| 0 | 160 | 5.63 | 32.3516 | 45.0477 | 0.253 |
| 0 | 160 | 10.99 | 29.7139 | 18.8270 | 0.291 |
| 1 | 10 | 6.28 | 7.7882 | 67.7785 | 0.232 |
| 1 | 30 | 7.92 | 16.4129 | 43.4066 | 0.274 |
| 1 | 430 | 83.24 | 24.4526 | 28.4995 | 0.274 |
| 1 | 420 | 140.83 | 41.9966 | 34.2412 | 0.260 |
| 1 | 400 | 140.83 | 41.9966 | 34.2412 | 0.260 |
| 1 | 500 | 0.50 | 7.5445 | 67.0780 | 0.240 |
| 1 | 470 | 7.84 | 19.3087 | 56.4031 | 0.253 |
| 1 | 110 | 21.15 | 23.4668 | 49.8908 | 0.262 |
| 1 | 460 | 24.82 | 21.3932 | 57.0430 | 0.243 |
| 1 | 450 | 3.11 | 4.8285 | 73.7518 | 0.216 |
| 1 | 40 | 6.66 | 7.4729 | 66.0061 | 0.240 |
| 1 | 160 | 5.63 | 32.3516 | 45.0477 | 0.253 |
| 1 | 160 | 10.99 | 29.7139 | 18.8270 | 0.291 |
| 0 | 160 | 10.99 | 29.7139 | 18.8270 | 0.291 |
| 1 | 500 | 0.50 | 7.5445 | 67.0780 | 0.240 |
| 1 | 460 | 24.82 | 21.3932 | 57.0430 | 0.243 |
| 1 | 450 | 3.11 | 4.8285 | 73.7518 | 0.216 |
| sp1 | sp2 | sp3 | sp4 | sp5 | sp6 | sp7 | sp8 | sp9 | sp10 | sp11 | sp12 | sp13 | sp14 | sp15 | sp16 | sp17 | sp18 | sp19 | sp21 | sp22 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Then, we perform a canonical correlation on the scaled tables \(R_{scaled}\) and \(C_{scaled}\). We find the coefficients \(\rho\) and \(\gamma\) maximizing the correlation between the scores \(S_R = R_{scaled} \rho\) and \(S_C = C_{scaled} \gamma\).
# Center tables
tabR_scaled <- scalewt(tabR, wt,
scale = FALSE)
tabC_scaled <- scalewt(tabC, wt,
scale = FALSE)
res <- cancor(diag(sqrt(wt)) %*% tabR_scaled,
diag(sqrt(wt)) %*% tabC_scaled,
xcenter = FALSE, ycenter = FALSE)
# res gives the coefficients of the linear combinations that maximizes the correlation between the 2 dimensions
dim(res$xcoef) # l columns -> R_scaled is of full rank[1] 6 6[1] 20 20We check that unique scores correspond:
For the scores computed with R scores and C scores, we have 3 types of formulas:
Here, we don’t show the definition.
Formulas using CCA scores
| Rows (sites) | Columns (species) | |
|---|---|---|
| Mean for axis \(k\) | \(m_k(i) = z2_{ik}\) ($l1) |
\(m_k(j) = v_{jk}\) ($c1) |
| Variance for axis \(k\) | None | \[s_{jk}^2 = \frac{1}{p_{\cdot j}} \sum_{i = 1}^r \left(p_{ij} {z2_{ik}}^2 - {v_{jk}}^2 \right)\] |
| Covariance between axes \(k\), \(l\) |
| Rows (sites) | Columns (species) | |
|---|---|---|
| Mean for axis \(k\) | \(u^\star_{ik}\) (WA score ls) |
\[v_{jk}\] ($c1) |
| Variance for axis \(k\) | \[s_k^2(i) = \frac{1}{p_{i \cdot}} \sum_{j = 1}^c \left(p_{ij} v_{jk} \right) - {u^\star_{ik}}^2\] | None |
| Covariance between axes \(k\), \(l\) |
In this section, we compute means and variances per row (for sites).
Below is a graphical illustration of scoreC grouped by rows:
s.class(scoreC,
wt = Pfreq0$Freq,
fac = as.factor(Pfreq0$row),
ppoints.col = params$colspp,
plabels.col = params$colsite,
main = "With C score (points = species scores)")
We compute the following mean: \(m_k(i) = \frac{1}{y_{i\cdot}} \sum_{j = 1}^c p_{ij} \text{scoreC}_k(i,j)\). To compute these conditional means, we use the meanfacwt function from the ade4 package.
Then we compare this to a score obtained from CCA.
Axis1 Axis2 Axis3 Axis4 Axis5 Axis6
1 1 1 1 -1 1 1
2 1 1 1 -1 1 1
3 1 1 1 -1 1 1
4 1 1 1 -1 1 1
5 1 1 1 -1 1 1
6 1 1 1 -1 1 1
7 1 1 1 -1 1 1
8 1 1 1 -1 1 1
9 1 1 1 -1 1 1
10 1 1 1 -1 1 1
11 1 1 1 -1 1 1
12 1 1 1 -1 1 1
13 1 1 1 -1 1 1
14 1 1 1 -1 1 1
15 1 1 1 -1 1 1
16 1 1 1 -1 1 1
17 1 1 1 -1 1 1
18 1 1 1 -1 1 1
19 1 1 1 -1 1 1
20 1 1 1 -1 1 1
21 1 1 1 -1 1 1
22 1 1 1 -1 1 1
23 1 1 1 -1 1 1
24 1 1 1 -1 1 1
25 1 1 1 -1 1 1
26 1 1 1 -1 1 1??????? axis 4 ?????
Now let’s compute the variance of scoreC per row:
We can use this relationship to find the formula using CCA scores:
varrowsC_CCA <- matrix(nrow = r,
ncol = neig)
for (i in 1:r) {
# Get CA scores for site i
Li <- cca$ls[i, ]
# Compute the part with the sum on Cj
# we add all coordinates Cj^2 weighted by the number of observations on site i
sumj <- t(P[i, ]) %*% as.matrix(cca$c1)^2
# sumj <- t(Y[i, ]) %*% as.matrix(cca$co)^2
# Fill i-th row with site i variance along each axis
# varrowsC_CCA[i, ] <- 1/(n*lambda_CCA)*((1/pi_[i])*sumj - lambda_CCA*as.numeric(Li)^2)
varrowsC_CCA[i, ] <- ((1/pi_[i])*sumj - as.numeric(Li)^2)
} X1 X2 X3 X4 X5 X6
1 1 1 1 1 1 1
2 1 1 1 1 1 1
3 1 1 1 1 1 1
4 1 1 1 1 1 1
5 1 1 1 1 1 1
6 1 1 1 1 1 1
7 1 1 1 1 1 1
8 1 1 1 1 1 1
9 1 1 1 1 1 1
10 1 1 1 1 1 1
11 1 1 1 1 1 1
12 1 1 1 1 1 1
13 1 1 1 1 1 1
14 1 1 1 1 1 1
15 1 1 1 1 1 1
16 1 1 1 1 1 1
17 1 1 1 1 1 1
18 1 1 1 1 1 1
19 1 1 1 1 1 1
20 1 1 1 1 1 1
21 1 1 1 1 1 1
22 1 1 1 1 1 1
23 1 1 1 1 1 1
24 1 1 1 1 1 1
25 1 1 1 1 1 1
26 1 1 1 1 1 1 X1 X2 X3 X4 X5 X6
1 1 1 1 1 1 1
2 1 1 1 1 1 1
3 1 1 1 1 1 1
4 1 1 1 1 1 1
5 1 1 1 1 1 1
6 1 1 1 1 1 1
7 1 1 1 1 1 1
8 1 1 1 1 1 1
9 1 1 1 1 1 1
10 1 1 1 1 1 1
11 1 1 1 1 1 1
12 1 1 1 1 1 1
13 1 1 1 1 1 1
14 1 1 1 1 1 1
15 1 1 1 1 1 1
16 1 1 1 1 1 1
17 1 1 1 1 1 1
18 1 1 1 1 1 1
19 1 1 1 1 1 1
20 1 1 1 1 1 1
21 1 1 1 1 1 1
22 1 1 1 1 1 1
23 1 1 1 1 1 1
24 1 1 1 1 1 1
25 1 1 1 1 1 1
26 1 1 1 1 1 1We can also compute the covariance between axes \(k\) and \(l\) for site \(i\) from CCorA scores:
\[c_{kl}(i) = \frac{1}{y_{i+}}\sum_{j=1}^c p_{ij}{s_Y}_k(i, j){s_Y}_l(i, j) - m_{ik}m_{il}\]
This is equivalent to the following formula using CCA scores:
\[ c_{kl}(i) = \frac{1}{n} \left( \sum_{j=1}^c p_{ij}v_{jk}v_{jl} - u^\star_{ik}u^\star_{il} \right)\]
Below, we compute the covariance for all resources between axes $k=$1 and $l = $2 and compare to the covariance computed from the weighted covariance of CCorA scores:
Now, we compute the mean per row (site) from the row (sites) scores.
Below is a graphical illustration of scoreR grouped by rows:
s.class(scoreR,
wt = Pfreq0$Freq,
fac = as.factor(Pfreq0$row),
ppoints.col = params$colsite,
plabels.col = params$colsite,
main = "With R score (points = sites scores)")
All points of the same site ares superimposed.
RS1 RS2 RS3 RS4 RS5 RS6
1 1 1 1 -1 1 1
2 1 1 1 -1 1 1
3 1 1 1 -1 1 1
4 1 1 1 -1 1 1
5 1 1 1 -1 1 1
6 1 1 1 -1 1 1
7 1 1 1 -1 1 1
8 1 1 1 -1 1 1
9 1 1 1 -1 1 1
10 1 1 1 -1 1 1
11 1 1 1 -1 1 1
12 1 1 1 -1 1 1
13 1 1 1 -1 1 1
14 1 1 1 -1 1 1
15 1 1 1 -1 1 1
16 1 1 1 -1 1 1
17 1 1 1 -1 1 1
18 1 1 1 -1 1 1
19 1 1 1 -1 1 1
20 1 1 1 -1 1 1
21 1 1 1 -1 1 1
22 1 1 1 -1 1 1
23 1 1 1 -1 1 1
24 1 1 1 -1 1 1
25 1 1 1 -1 1 1
26 1 1 1 -1 1 1The variance is null.
We group the scoreC per species. Below is an illustration:
s.class(scoreC,
wt = Pfreq0$Freq,
fac = as.factor(Pfreq0$colind),
lab = colnames(Y),
ppoints.col = params$colspp,
plabels.col = params$colspp,
main = "With C score (points = species scores)")
CS1 CS2 CS3 CS4 CS5 CS6
sp1 1 1 1 -1 1 1
sp2 1 1 1 -1 1 1
sp3 1 1 1 -1 1 1
sp4 1 1 1 -1 1 1
sp5 1 1 1 -1 1 1
sp6 1 1 1 -1 1 1
sp7 1 1 1 -1 1 1
sp8 1 1 1 -1 1 1
sp9 1 1 1 -1 1 1
sp10 1 1 1 -1 1 1
sp11 1 1 1 -1 1 1
sp12 1 1 1 -1 1 1
sp13 1 1 1 -1 1 1
sp14 1 1 1 -1 1 1
sp15 1 1 1 -1 1 1
sp16 1 1 1 -1 1 1
sp17 1 1 1 -1 1 1
sp18 1 1 1 -1 1 1
sp19 1 1 1 -1 1 1
sp21 1 1 1 -1 1 1
sp22 1 1 1 -1 1 1The variance is null.
Below are the scoreR grouped by column:
s.class(scoreR,
wt = Pfreq0$Freq,
fac = as.factor(Pfreq0$colind),
lab = colnames(Y),
ppoints.col = params$colsite,
plabels.col = params$colspp,
main = "With R score (points = sites scores)")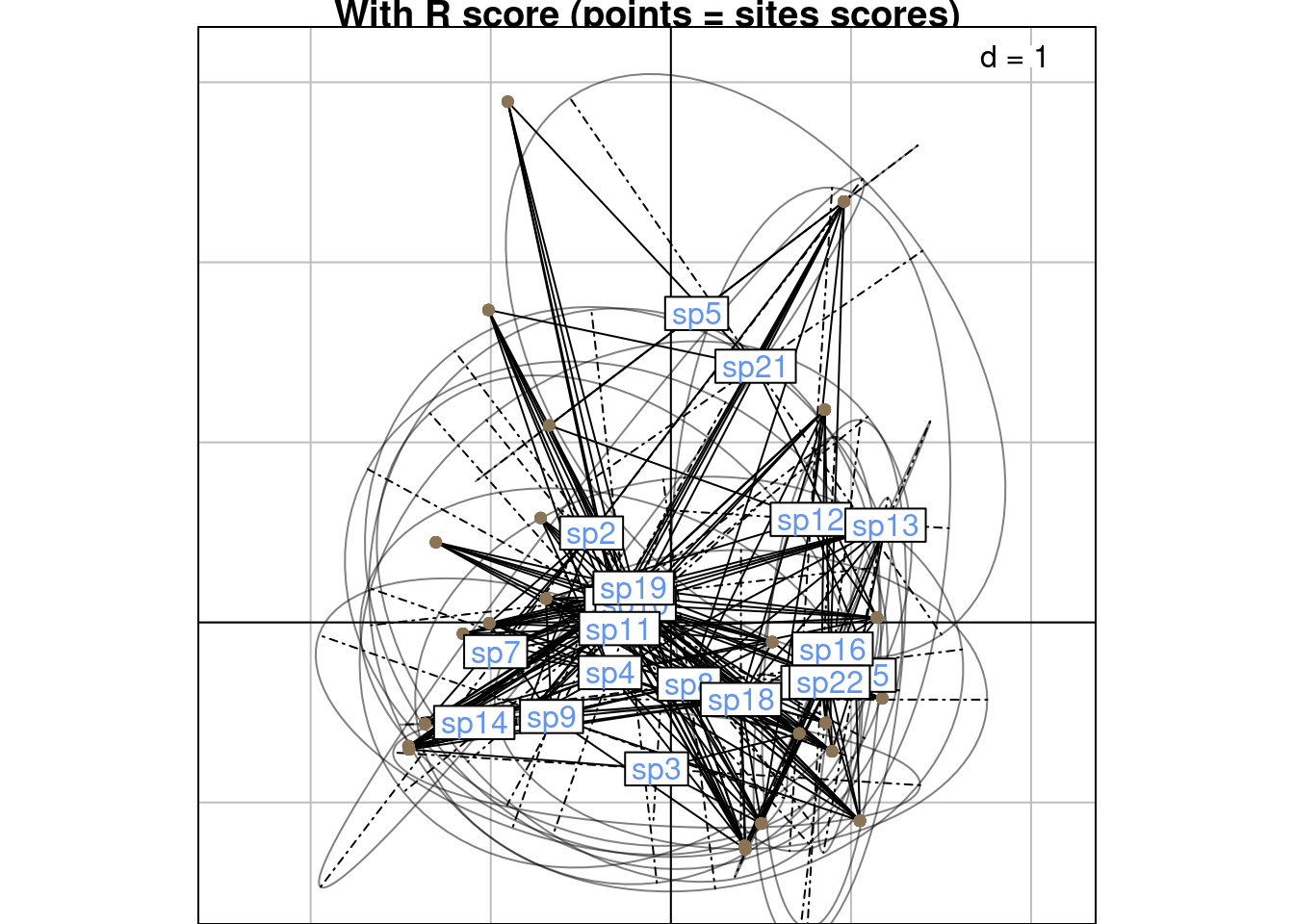
Comp1 Comp2 Comp3 Comp4 Comp5 Comp6
sp1 1 1 1 -1 1 1
sp2 1 1 1 -1 1 1
sp3 1 1 1 -1 1 1
sp4 1 1 1 -1 1 1
sp5 1 1 1 -1 1 1
sp6 1 1 1 -1 1 1
sp7 1 1 1 -1 1 1
sp8 1 1 1 -1 1 1
sp9 1 1 1 -1 1 1
sp10 1 1 1 -1 1 1
sp11 1 1 1 -1 1 1
sp12 1 1 1 -1 1 1
sp13 1 1 1 -1 1 1
sp14 1 1 1 -1 1 1
sp15 1 1 1 -1 1 1
sp16 1 1 1 -1 1 1
sp17 1 1 1 -1 1 1
sp18 1 1 1 -1 1 1
sp19 1 1 1 -1 1 1
sp21 1 1 1 -1 1 1
sp22 1 1 1 -1 1 1Similarly we can use this relationship to compute the variance directly from the CCA scores (see in table).
We can also compute the covariance between axes \(k\) and \(l\) for species \(j\) from CCorA scores:
\[c_{kl}(j) = \frac{1}{y_{+j}}\sum_{i=1}^r p_{ij}{s_R}_k(i, j){s_R}_l(i, j) - m_{jk}m_{jl}\]
This is equivalent to the following formula using CCA scores:
\[c_{kl}(j) = \frac{1}{n} \left( \sum_{i=1}^r p_{ij}z2_{ik}z2_{il} - v^\star_{jk}v^\star_{jl} \right)\]
Below, we compute the covariance for all resources between axes $k=$1 and $l = $2 and compare to the covariance computed from the weighted covariance of CCorA scores:
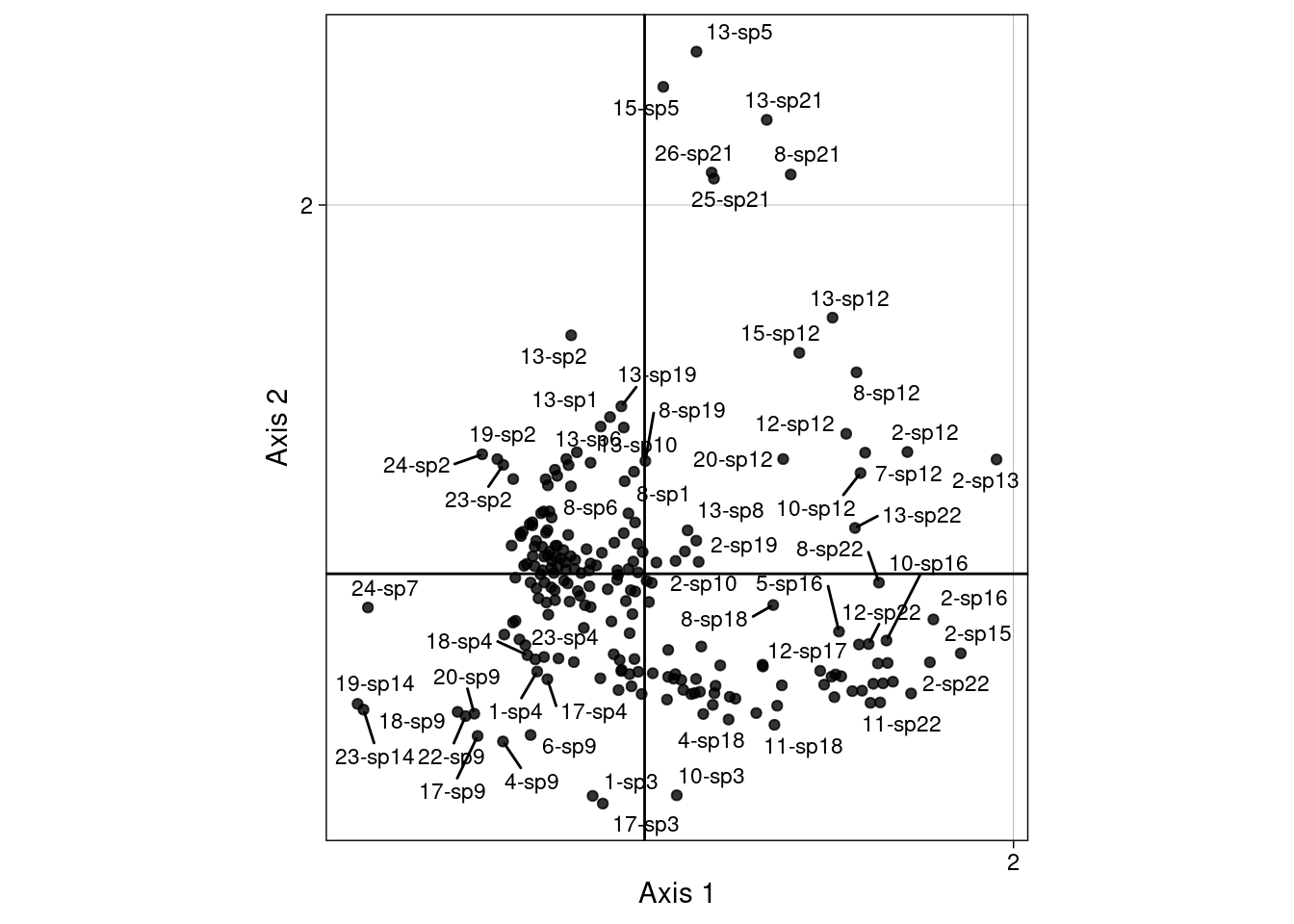
We can remain in the 2 biplots showing \(V\) with \(U^\star\) (c1 and ls) (scaling type 1 = rows niches) and \(U\) and \(V^\star\) (co and l1) (scaling type 2 = column niches).
To do that, we define the “CA-correspondences” \(h1_k(i, j)\) for scaling type 1:
\[h1_k(i, j) = \frac{u^\star_k(i) + v_k(j)}{2}\]
# Initialize results matrix
H1 <- matrix(nrow = nrow(Pfreq0),
ncol = neig)
for (k in 1:neig) { # For each axis
ind <- 1 # initialize row index
for (obs in 1:nrow(Pfreq0)) { # For each observation
i <- Pfreq0$row[obs]
j <- Pfreq0$col[obs]
H1[ind, k] <- (cca$ls[i, k] + cca$c1[j, k])/2
ind <- ind + 1
}
}
# Groupwise mean
mrows_nr <- meanfacwt(H1, fac = Pfreq0$row, wt = Pfreq0$Freq)
all(abs(abs(mrows_nr/cca$ls) - 1) < zero)[1] TRUE# Groupwise variance ---
# Compare to RC score
varrows_nr <- varfacwt(H1, fac = Pfreq0$row, wt = Pfreq0$Freq)
# Compare to mean of scoreC
res <- 4*varrows_nr # Factor 4
all(abs(abs(res/varrowsC[, 1:neig]) - 1) < zero)[1] TRUE# Groupwise covariance
covarrows_nr <- covfacwt(H1, fac = Pfreq0$row, wt = Pfreq0$Freq)
res <- lapply(covarrows_nr, function(x) x*4) # Factor 4
# We check for the first covariance
all(abs(abs(res[[1]]/covarrowsC[[1]][1:l, 1:l]) - 1) < zero)[1] TRUE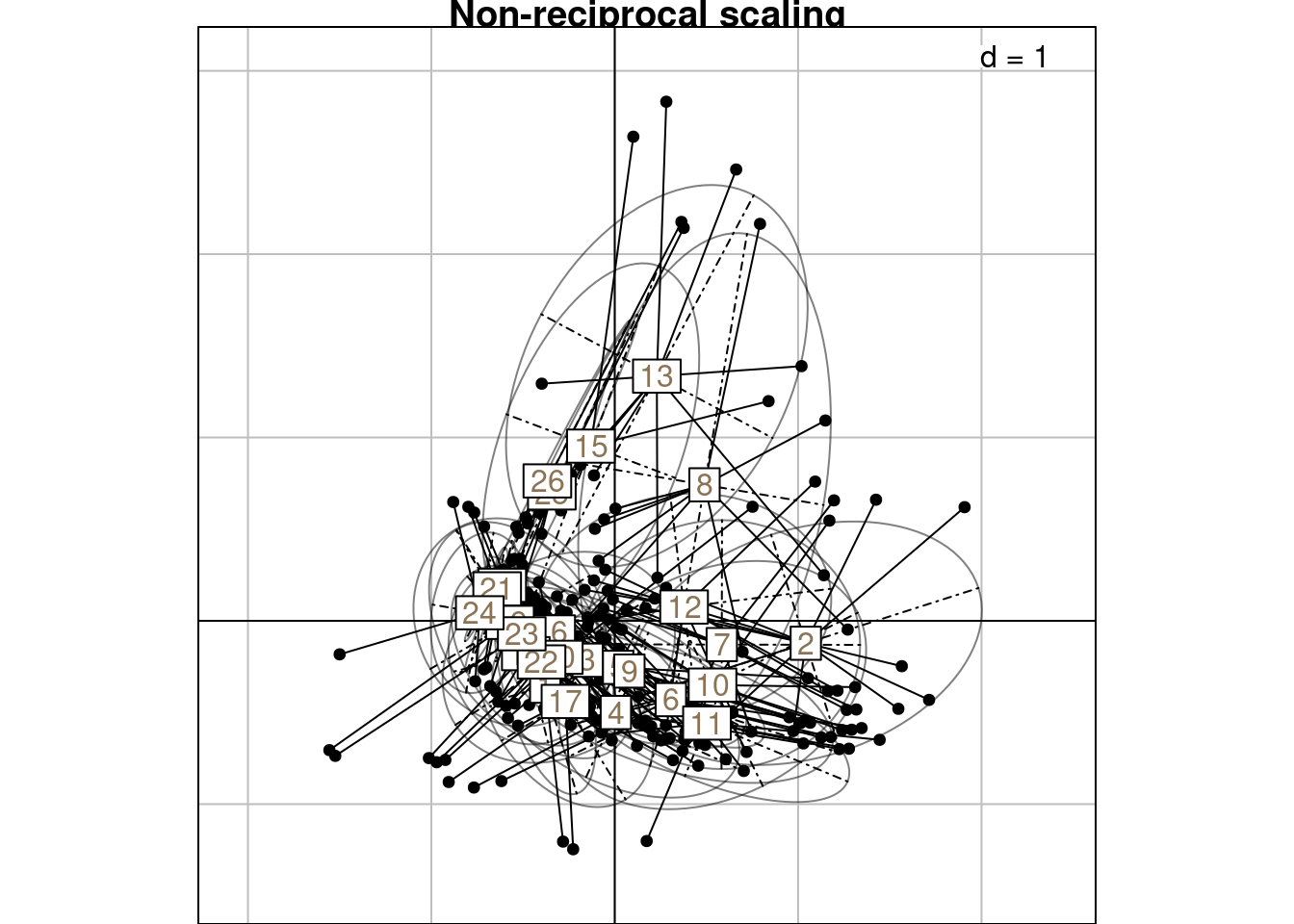
\(h1_k(i, j)\) can also be defined from the canonical correlation scores:
\[h1_k(i, j) = \frac{m_{ik} + {s_Y}_k(i, j)}{2}\]
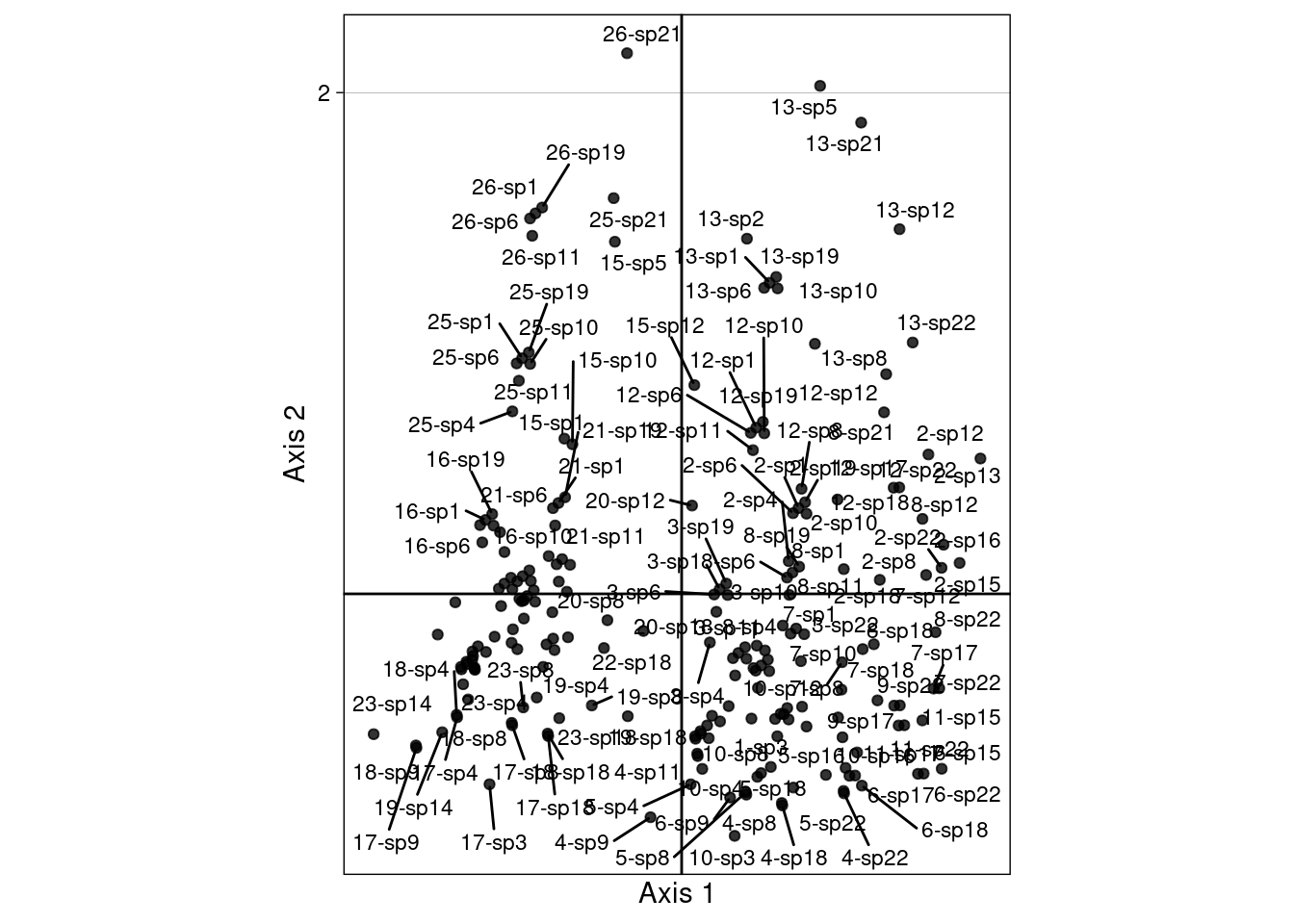
We define the “CA-correspondences” \(h2_k(i, j)\) for scaling type 2:
\[h2_k(i, j) = \frac{z2_k(i) + v^\star_k(j)}{2}\]
# Initialize results matrix
H2 <- matrix(nrow = nrow(Pfreq0),
ncol = neig)
for (k in 1:neig) { # For each axis
ind <- 1 # initialize row index
for (obs in 1:nrow(Pfreq0)) { # For each observation
i <- Pfreq0$row[obs]
j <- Pfreq0$col[obs]
H2[ind, k] <- (cca$l1[i, k] + cca$co[j, k])/2
ind <- ind + 1
}
}
# Groupwise mean ---
mcols_nr <- meanfacwt(H2, fac = Pfreq0$col, wt = Pfreq0$Freq)
# Compare with CCA score
all(abs(abs(mcols_nr/cca$co) - 1) < zero) [1] TRUE
\(h2_k(i, j)\) can also be defined from the canonical correlation scores:
\[h2_k(i, j) = \frac{ {s_R}_k(i, j) + {s_Y}_k(i, j) \sqrt{\lambda_k} }{2}\]